Globally Renowned Products
Our innovative Rapid Diagnostic Test (RDT) platforms simplify procedures, mitigate common user errors and enhance test performance. Our products are currently approved for distribution in over 40 countries.
Atomo Diagnostics HIV Self Test
Atomo has created the world's first integrated, blood-based rapid diagnostic test (RDT) for HIV screening, renowned globally for its innovative design, performance, and user-friendliness.
In contrast to traditional clinical testing, the Atomo HIV Self Test delivers results in just 15 minutes. This rapid HIV test boasts a remarkable accuracy, with a specificity and sensitivity of 99.6%, thanks to interlocked user steps that make it easy for lay users to perform the test comfortably and discreetly in private settings.
With effective and easily accessible treatment medications available in Australia, it is crucial to offer multiple pathways for HIV testing.
Atomo's HIV Self Test provides an alternative testing method for individuals who may face stigma or other barriers that discourage them from visiting sexual health clinics or medical facilities for HIV testing.

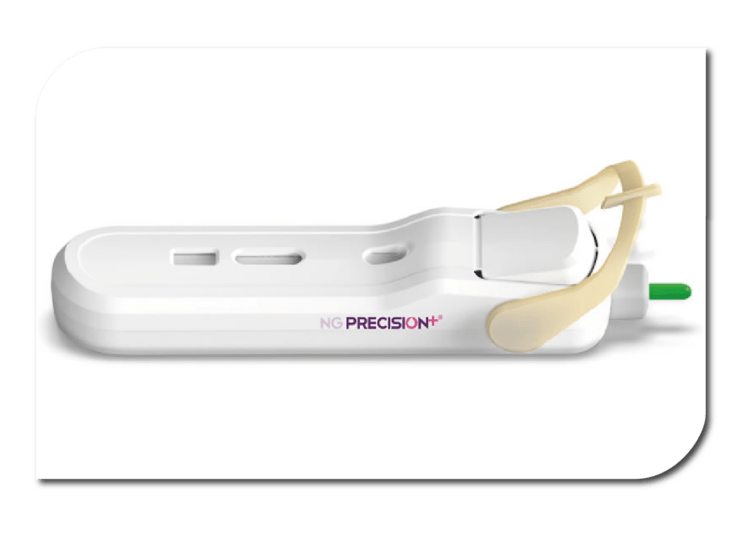
NG Biotech Precision+ Pregnancy Test
NG Biotech is an innovative French biotechnology company specialising in the development and manufacturing of cutting-edge in vitro diagnostic tools for therapy monitoring at the point of care. By focusing on onsite diagnostic solutions, NG Biotech aims to enhance patient care through timely and accurate monitoring, ensuring that healthcare providers can make informed decisions quickly and efficiently.

NG's Precision+ is the first all-in-one at-home blood pregnancy test designed for rapid and reliable results. Widely used in professional settings, NG PRECISION+ revolutionises pregnancy testing by offering a dependable and immediate diagnosis. Its practical and hygienic design allows for use at any time, ensuring convenience and accuracy.
The NG Precision+ is available for purchase across Europe.
Lumos Diagnostics FebriDx™
Lumos Diagnostics specialises in rapid, cost-effective point-of-care (POC) diagnostic solutions, aiding healthcare professionals in accurately diagnosing and managing medical conditions. Lumos offers custom assay development and manufacturing services for POC tests, along with proprietary digital reader platforms. Additionally, Lumos develops, manufactures, and markets its own branded POC tests targeting infectious and inflammatory diseases.
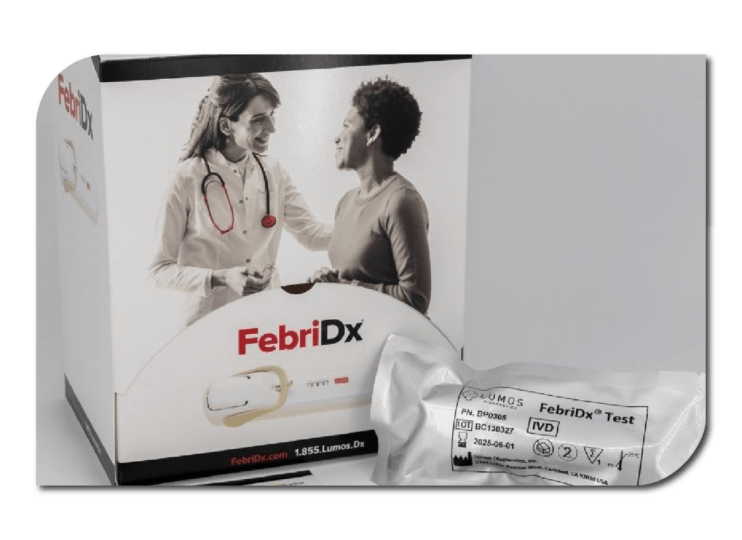
Using Atomo's Pascal platform, Lumos' FebriDx platform is available in Europe and has received FDA clearance for the U.S. market. This test aids in identifying and differentiating between viral and bacterial acute respiratory infections. With 99% accuracy, FebriDx has reduced antibiotic prescriptions by 80%, resulting in improved efficiency and lower costs.
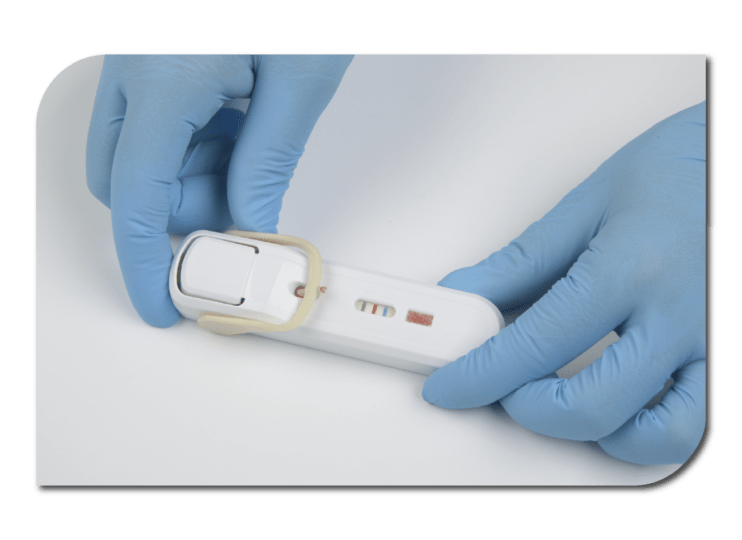
With overwhelming user preference, Atomo's unique and innovative solutions are leading the way

90%
Reduction in Blood Delivery Errors

100%
Reduction in Buffer Delivery Errors

40%
Reduction in Procedure Time









