Our Product Development Process
Our aim is to change how diagnostic testing is performed in both point of care settings and in the home. We are a world leader rapid test manufacturer creating integrated, user friendly rapid diagnostic tests platforms.
Atomo has an established track record of working with OEM partners in the rapid diagnostic manufacturing space, such as NG Biotech and Lumos Diagnostics, to deliver quality products and solutions.

“Atomo has created a product that addresses unmet user needs in POC testing with superior accuracy, usability and simplicity.”
Milovan Stankov Puges
CEO, NG Biotech
With the growing demand for point of care and at-home rapid diagnostics, Atomo can be a launching pad for any lateral flow assay company to diversify and improve their current offerings by leveraging over a decade’s worth of R&D to produce tests that are light years ahead of industry standards.
Our solutions are preferred by 90% of users due to greatly improved usability and reduced propensity for error.
Custom Product Development
Atomo’s robust product development process is compliant to ISO 13485 and provides a rapid and effective route from design to commercialisation.
Atomo uses a phase gate approach product development process with design reviews at the end of each stage and can support a robust documentation package to support regulatory submissions. Atomo’s team of engineers will work directly with lateral flow manufacturers and assay developers. We strive to understand your rapid test requirements and look to eliminate pain points.
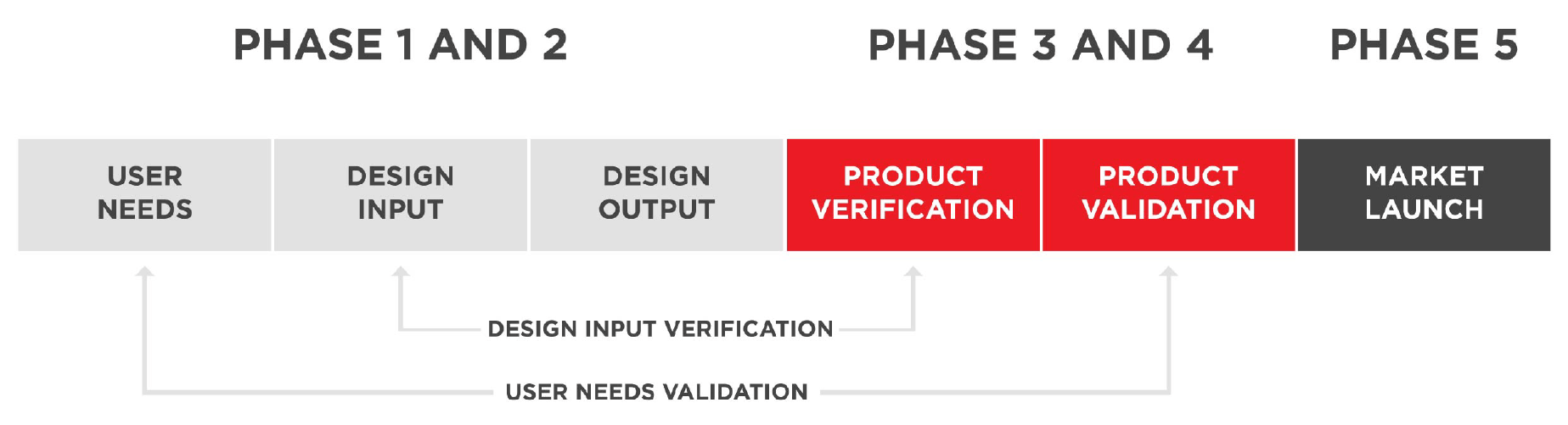
Our Product Development Pathway
At Atomo Diagnostics, we understand that innovation often requires unique solutions. Our custom product development services are designed to bring your visionary ideas to life. Whether you’re developing a novel biomarker test, exploring multi-analyte platforms, or creating specialised diagnostic tools for emerging diseases, our team of expert engineers is ready to collaborate. Our agile approach allows us to tackle challenges at any stage of development, from initial concept to final product refinement.
We offer a range of proven integrated test cassettes backed by regulatory approvals in key international markets, including the U.S., as well as offering custom solutions based on our unique, user-focused proprietary blood and swab-testing technology.

Co-Development
Collaboration throughout the entire development process, from assay optimisation to final product launch

OEM Partnerships
Leverage expertise in usability, manufacturing and regulatory affairs to bring assays to market

Technology Licensing
Licensing options for proprietary technologies, including unique blood collection systems and integrated buffer handling

Custom Design
Solutions tailored to exact specifications, from modification of existing cassettes to new device concepts
Design, Prototyping and Pilot Production
While each platform differs to a degree in its operational capacity, each device in the AtomoRapid™ cassette range includes patented rapid diagnostic test platforms that meet requirements for professional use and self-test applications. The first step of the partnership development pathway is the selection of the appropriate rapid diagnostic test platform. To do this, we conduct a full analysis of your user requirements, with research centring on human factors and user testing to ensure the solution we determine is most appropriate for your needs. We then undertake concept feasibility studies in order to progress to the development of functional prototypes before assisting with the management of intellectual property.

Verification and Validation
Each platform in the AtomoRapid™ range is customisable to suit your analyte and test strip accordingly. Each platform has full clinical validation and comes with company-wide quality assurance. We utilise an FMEA manufacturing process analysis and can assist and support with regulatory submissions.

Commercial Launch and Rollout
Our commercial launch and rollout process involves full manufacturing process validation. All of our products are manufactured either at our own high quality manufacturing and assembly facilities or by our trusted subcontractors. As part of our commercialisation process, we offer a full suite of brand, packaging, and marketing assistance. Once your product is launched, our commercial team can provide continued post-launch support to ensure the best chance of success.

With Atomo as your partner, you gain access to cutting-edge technology and a wealth of experience in overcoming the hurdles of custom diagnostic test development.





